The data was recorded in table 92. The NiSO 4 concentration in molL will be equal to the corresponding X value.
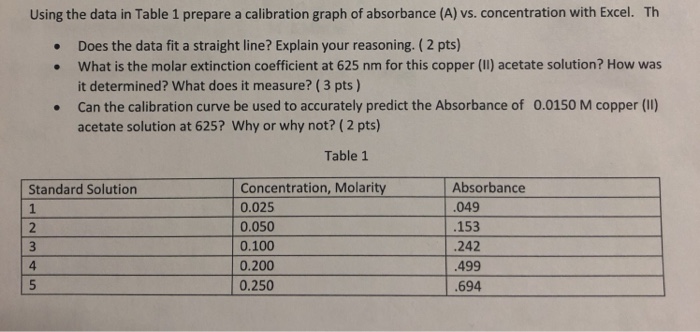
Solved Using The Data In Table 1 Prepare A Calibration Graph Chegg Com

Standard Curve An Overview Sciencedirect Topics

Absorbance And Calculated Concentration Obtained For Dissolution Test Download Table
When you take an absorbanc spectrum the molar absorptivity constant is changing up and down and all around but when you are working with a calibration plot and are only changing the concentration it shows a linear relationship between.
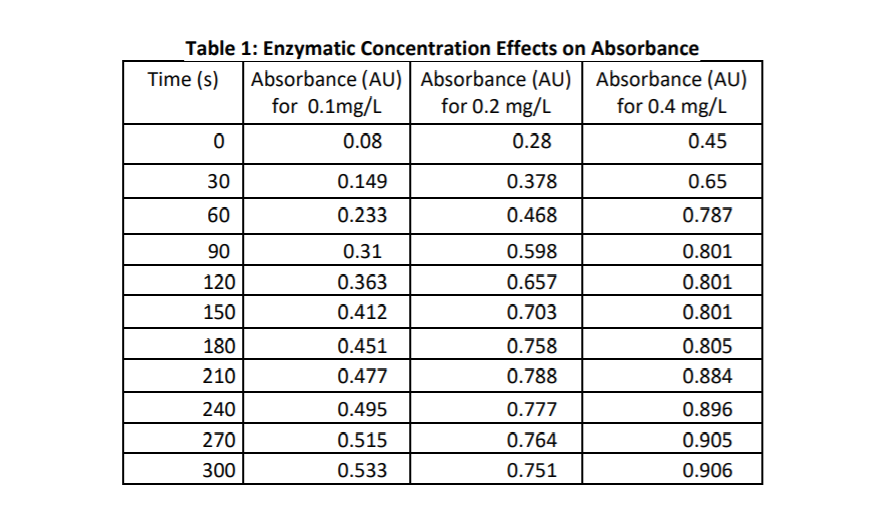
Table of of absorbance vs. Include the equation of the line on the plot. Data sheet 1 1- Place the data of dissolution test of Paracetamol tablet then plot the amount released vs time Time Absorbance Concentration of mgml min Amount released mg 5 10 15 20 25 0353 0463 0517 0481 0510. Absorbance vs Transmittance.
In analytical chemistry a calibration curve also known as a standard curve is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. Summarize your absorbance data for the standard curve in a well labeled table. 6 Measure the absorbance of the solution of unknown concentration using the colorimeter.
For example 10Au is equal to 10 transmittance 20Au is equal to 1 transmittance and so on in a logarithmic trend. Since concentration and absorbance are proportional Beers Law makes it possible to determine an unknown concentration of phosphate after determining the absorbance. For instance the absorbance of the alkene 2-methyl pent-2-ene is below 200 nm as is the ππ absorbance of 4-methyl pentane-2-one below.
The true unit of measurement of absorbance is reported as absorbance units or AU. Record this value in your data table. 32 Attach an Excel plot of Absorbance vs.
Cursor to an absorbance value Y value that is closest to the absorbance reading you obtained in Step 1 0. Print your graph once it is titled Y vs X includes the equation of the line and the R2 value has a label for each axis and includes any appropriate units. Determine the equation for the line of A vs C.
Table 91 This table represents the measures of color intensity of betacyanin leaked from damaged cells treated at six different temperatures. Click entering a concentration of 0. The plot must have labeled axes title equation of the line and R2 value.
Gives 0000 absorbance based on the absorbance at 275 nm of protein sample. Print a graph of absorbance vs. Your first data point will be the blank since the absorbance is 0 in the absence of Allura Red.
Table 4 below shows the typical layout of a table in three sections demarcated by lines. You are now ready to collect absorbance data for the four standard solutions. The overall goal of this lab was to make a calibration curve with a plot of absorbance vs.
On your calculator this is designated as ln. One caveat of using absorbance based measurements of nucleic acid samples is that proteins and reagents commonly used in the preparation of nucleic acids also absorb light at 260 nm and can lead to falsely elevated concentration results. A calibration curve is one approach to the problem of instrument calibration.
Answer to Solved Old MathJax webview Transcribed image text. Y-03328 -009US Absorbance of brass sampleL 329 4 M AT Post-lab questions. Concentration using data from the table.
This is the calibration curve. The difference between these two terms is summarized in the table below. Using the measured absorbance values for the working standards listed as samples no.
Concentration with a regression line and interpolated unknown concentration displayed. Blinks the absorbance should read 0 or near 0. If you measure the absorbance of your solution to be A 0089 when d 1 cm then gL mg L d g Lcm cm g L A C 000445.
Average the four absorbance values for each KBr pellet sample. Then once you have that you can put in your value for A of the unknown sample. In mesityl oxide where the alkene and CO group are in conjugation with each other the absorption maximum moves to longer wavelength at 228 nm.
The best way to recognize the difference between laid and lain is to remember that they belong to two different verbs. Again in the same table find the inverse reciprocal of each absorbance value. Plot using the graph paper provided a graph of 1Abs versus Time.
The method shows imprecision CV35 49 Table 1 and inaccuracy AvDev 576 Table 3The fading of colour during reading of absorbance adds to this imprecision and inaccuracy. If the peak at 800 cm-1. 7 Measure absorbance of standard solutions to create a calibration plot.
Which method is used Table 5. Tables are most easily constructed using your word processors table function or. Add a line to your data.
Concentrations of albumin as shown in the table. The absorbance was also recorded by an absorbance recording machine. Ples and the five standard solutions is shown in Table 1.
Is small expand the ordinate to enhance the peak height. Absorbance is measured in absorbance units Au which relate to transmittance as seen in figure 1. Print the polished graph and staple it.
Transmittance is a ratio of the incident intensity of light. 3 was too high for the spectrophotometer to recognize at the specified settings. 3 Measure the absorbance of each standard solution using the colorimeter.
Absorbance and transmittance both terms are opposite to each other. 445 20. Calibration curves of integrated fluorescence intensity area against absorbance for rhodamine 6G red and rhodamine B black.
Absorbance and integrated fluorescence intensities areas for rhodamine 6G standards and rhodamine B samples. Use this graph to determine the unknown concentrations of two samples of the protein showing your work. Concentration and be able to determine the phosphate concentrations in samples of cola surface water and other aqueous solutions of.
Key Difference Laid vs Lain Laid and lain are two past participle verbs that confuse native and non-native English speakers alike. Antibody conjugation is usually achieved through chemical reaction although specific enzymatic conjugation such as sortase-based protein terminal conjugation 1 2 or even through protein engineering is gaining popularityThe preferred site for chemical conjugation on the antibody is the NH 2 amine group of a lysine or the free SH sulfhydryl group of cysteine. Wavelength molar absorptivity constant and the calibration plot is vs.
We quantified the relative color of each solution between 0 colorless and 10 darkest red. If the sample was ashed at low temperature Step 4a the presence of kaolinite will be indicated by an absorption band with maximum at 915 cm-1. Starch mg for the standard solutions.
Use Excel to prepare a graph of A 620 nm vs. Plot using the graph paper provided a graph of lnAbs versus Time. Transmittance When something is absorbed it means that that substance in this case light is essentially captured by an object and not able to escape.
The Modified Folin Wu method though sensitive is nonlinear. Note that the absorbance for sample no. 4 Plot the absorbance vs concentration for each standard solution on a graph.
Other standard approaches may mix the standard into the unknown giving. The Anatomy of a Table. Automatic peak integration using the relative quantum yield module.
5 through 10 in Table 1 the standard curve shown in Figure 2 was developed. Equation of the line. 3 5 Draw the line of best fit through the data points.
Draw an appropriate baseline under the. Both are plotting absorbance but the spectrum plots it vs. What unit is absorbance used.
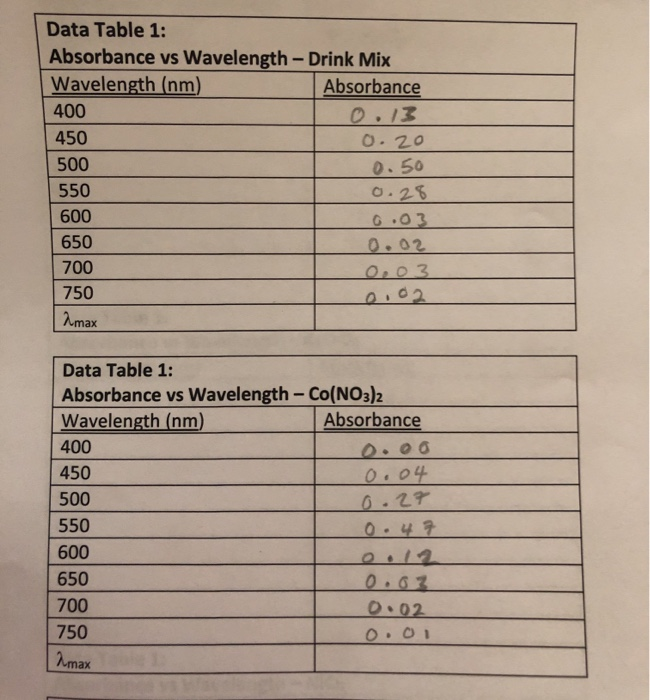
Solved Data Table 1 Absorbance Vs Wavelength Drink Mix Chegg Com

Solved The Table Above Shows Absorbance Values For Varying Chegg Com
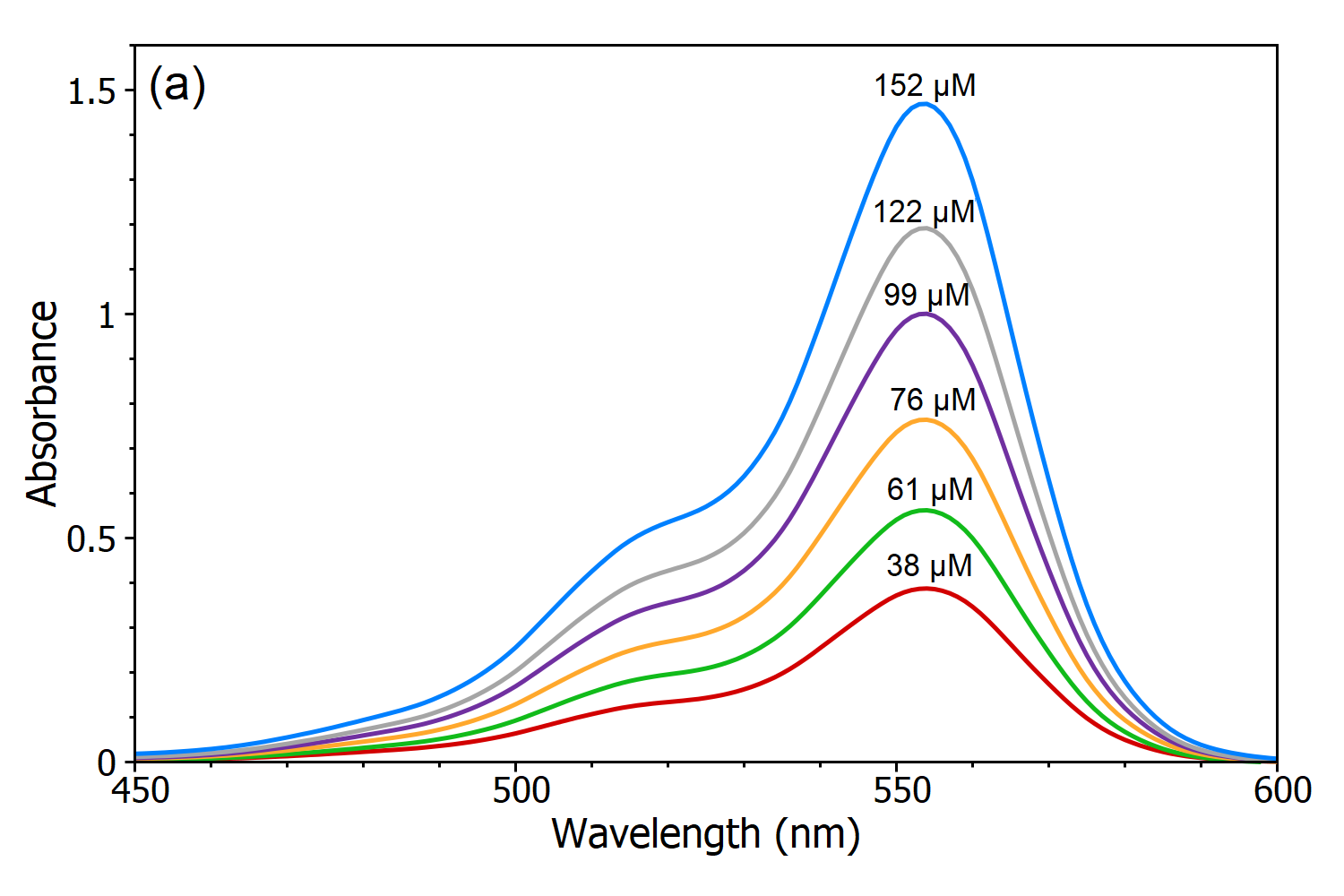
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
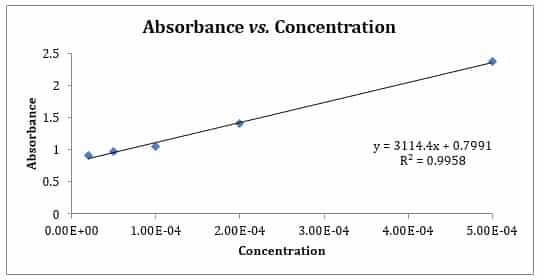
Beer S Law Lab Explained Absorbance Vs Concentration Schoolworkhelper
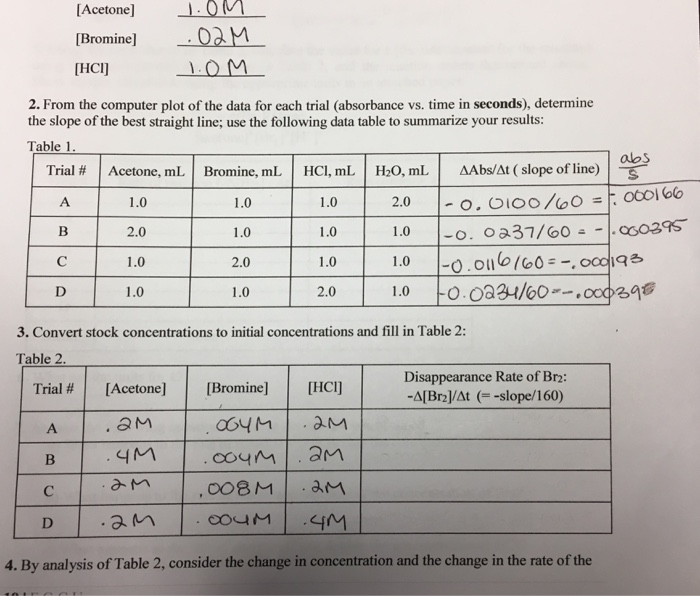
Solved Acetone Bromine Hci 2 From The Computer Plot Chegg Com
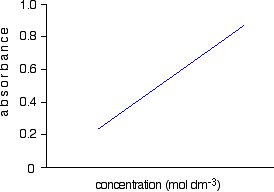
Using Uv Visible Absorption Spectra

Table 4 From Uv Visible Absorbance Spectroscopy As A Proxy For Peatland Dissolved Organic Carbon Doc Quantity And Quality Considerations On Wavelength And Absorbance Degradation Semantic Scholar
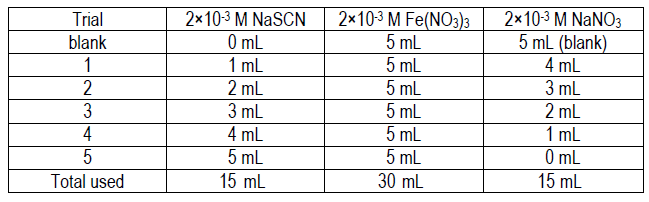
Lab 11 Spectroscopic Determination Of An Equilibrium Constant